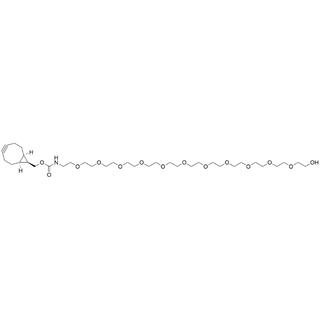
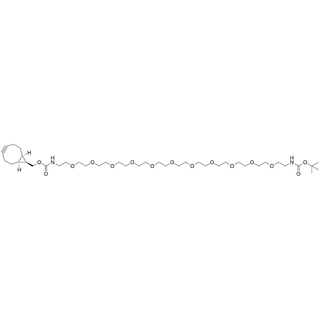
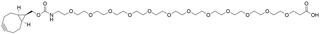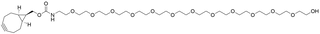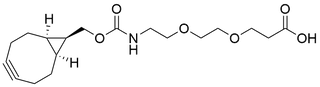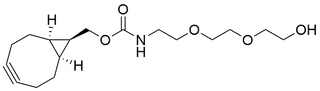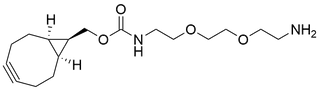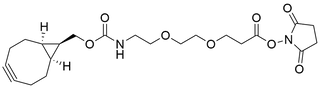- Products
- PEG Linkers
- ADC Linkers and Linker Attachment
- Chemical Proteomics Reagents
- Click Chemistry Reagents
- Targeted Protein Degrader Building Blocks
- Biotinylation Reagents
- Radionuclide Drug Conjugates (RDCs)
- PEG Linkers
- View all...
- Acrylate PEG
- Alcohol PEG
- Amino PEG
- Aminooxy PEG
- Aryl PEG
- Azido PEG
- BCN PEG
- Biotin PEG
- Bromo PEG
- Bromoacetamide PEG
- DBCO PEG
- DNP PEG
- DOTA PEG
- DOTAGA PEG
- m-PEG
- Maleimide PEG
- PEG Acid
- PEG Aldehyde
- PEG CH2COOH Acid
- PEG Fluorescent Dye
- PEG Hydrazide
- PEG Mesylate
- PEG NHS Ester
- PEG PFP Ester
- PEG Phosphonate
- PEG Phosphonic Acid
- PEG SDP Ester
- PEG Silane
- PEG Sulfonic Acid
- PEG TFP Ester
- PEG Tosylate
- Propargyl PEG
- SPDP PEG
- TCO PEG
- Tetrazine PEG
- Thiol PEG
- Mono-dispersed PEG2000 (PEG45)
- Branched PEG
- Amine reactive PEG derivatives
- Bifunctional x-Arm PEG
- Biotinylation PEG derivatives
- Carbonyl reactive PEGs
- Carboxyl reactive PEGs
- Click chemistry PEGs
- DOTA chelator PEGs
- Drug delivery PEGs
- Dye labeling PEGs
- Nanoparticle PEGylation
- Peptide and Protein PEGylation
- Polymerizable PEGs
- Specialty PEGylation reagents
- Surface Reactive PEG Derivatives
- Thiol reactive PEG derivatives
- Thiol-ene Crosslinker Chemistry
PEG Derivatives by Functional Group (Functionality)
PEG Derivatives by Application (Reactivity)
- ADC Linkers and Linker Attachment
- View all...
- Bispecific Antibodies (BsAbs) Linker
- Cleavable Linkers
- Non-Cleavable Linkers
- Linker Attachment
- Linker-Payload
- Chemical Proteomics Reagents
- View all...
- Crosslinker
- Photoaffinity Labeling
- Other Chemical Proteomics Reagents
- Targeted Protein Degrader Building Blocks
- View all...
- CRBN Ligand-Linker Conjugate
- Other Ligands-Linker Conjugate
- VHL Ligand-Linker Conjugate
- Biotinylation Reagents
- View all...
- Uncleavable Biotinylation Reagents
- Cleavable Biotinylation Reagents
- Desthiobiotin/Biotin Sulfoxide/Biotin Sulfone
- Radionuclide Drug Conjugates (RDCs)
- View all...
- Deferoxamine Derivatives
- DTPA
- RDC DOTA
- RDC DOTAGA
- NOTA/NOTAGA/Other Chelators
- Targeting Ligands
We've sent you an email with a link to update your password.
Login
Reset your password
We will send you an email to reset your password.
By completing this form, you are signing up to receive our emails and can
unsubscribe at any time.
Managed By Digital Marketing INC